One of the most exciting areas in clinical neuroscience is the development of neuromodulation interventions for the treatment of neurological and psychiatric disorders. There are generally 2 major categories of neuromodulation: invasive and non-invasive. Some examples of invasive forms of neuromodulation include Deep Brain Stimulation which has been FDA approved for the treatment of Parkinson’s disease and requires neurosurgery. Implanted neuromodulators are also FDA approved for the treatment of chronic pain. These devices require surgery to implant the neuromodulation devices to block or attenuate pain messages, usually at the spinal cord level. Electrical Convulsive Therapy or ECT, is also considered invasive because it creates a generalized seizure and patients are anesthetized during the procedure. Where ECT requires 800 – 1,000 milliamps, non-invasive neuromodulation intervention uses, in adults, typically up to 2 milliamps and typically up to 1 milliamp in children.
Only non-invasive neuromodulation (NM) interventions are used at NCBI. These interventions do not require any surgery or anesthesia. NM works typically by applying weak electrical currents to influence or modulate neurophysiological activity in the brain. These currents are generally applied from outside of the head, i.e., transcranial, through EEG sensors that rest on the surface of the scalp. Other common targets are the cranial nerves, accessed from the forehead or outer ear.
In several countries, including the USA, various forms of NM interventions have been approved for the treatment of depression and chronic pain. Many NM studies have also been conducted to study cognitive enhancement in both healthy non-clinical study volunteers as well as in clinical populations such as Alzheimer’s disease, stroke, ADHD, Autism, and traumatic brain injury. NM is easy to apply, considered to be safe and only mild adverse effects have been reported. These typical consist of mild tingling and light itching under the electrodes. In all the NM studies conducted to date – there has never been a serious adverse event resulting in any hospitalization, permanent injury or death.
Although many NM medical devices are new and the FDA recently approved some for conditions such as ADHD, Opiate withdrawal, chronic pain and migraine, others such as CES devices were approved in the 1970s by the FDA for treatment of anxiety/insomnia and depression. However, treatment results with many of these older medical devices have not been that effective. Research is being conducted here at NCBI and the NCBI Clinical Research Foundation and around the world investigating if newer neuromodulation medical devices are more effective for a host of neurological and psychiatric disorder.
Among these various neuromodulation applications, Neurofeedback is the only active form of neuromodulation requiring the patient to learn to gain control over specific neurophysiological activity. Although Neurofeedback has been used as a clinical tool since the 1960s, brain mapping neurofeedback using LORETA software is a relatively recent development dating back to approximately 2008. This technology uses advancements made over the past 20 years mapping multiple brain circuits responsible for a plethora of human experiences; from basic functions such as sustaining wakefulness to complex functions such as decision-making. Watch video
Among the transcranial NM interventions, transcranial direct current stimulation (tDCS) has been the most widely studied over the past 25 years. This is a non-invasive, painless brain modulation treatment that uses very small direct electrical currents to stimulate specific parts of the brain. A constant, low intensity current is passed through two or more electrodes placed over the head which modulates neuronal activity. Several hundred studies suggest it may be a valuable tool for the treatment of cognitive disorders, as well as, neuropsychiatric conditions such as mood disorders and chronic pain. Although approved in several countries for the treatment of depression and pain, currently, tDCS is not an FDA-approved treatment yet in the US but in several other currents, tDCS has been approved to treat depression. Learn more
Transcranial Alternate Current Stimulation (tACS) is similar to tDCS, but instead of applying a direct electrical current, tACS oscillates a sinusoidal current at a chosen frequency to interact with the brain’s natural cortical oscillations. The advantage of this approach is the entrainment of cortical networks or circuits which can manipulate specific frequencies to slow down or enhance activity in these networks or regions. tACS is a very effective treatment for several cognitive disorders as a standalone therapy, in conjunction with cognitive remediation and is often combined with monosinusoidal tDCS and tRNS (random noise stimulation) to treat:
ADHD
Executive dysfunction from multiple etiologies
Intellectual disorders
Alzheimer’s disease
Aphasia and paralysis from stroke
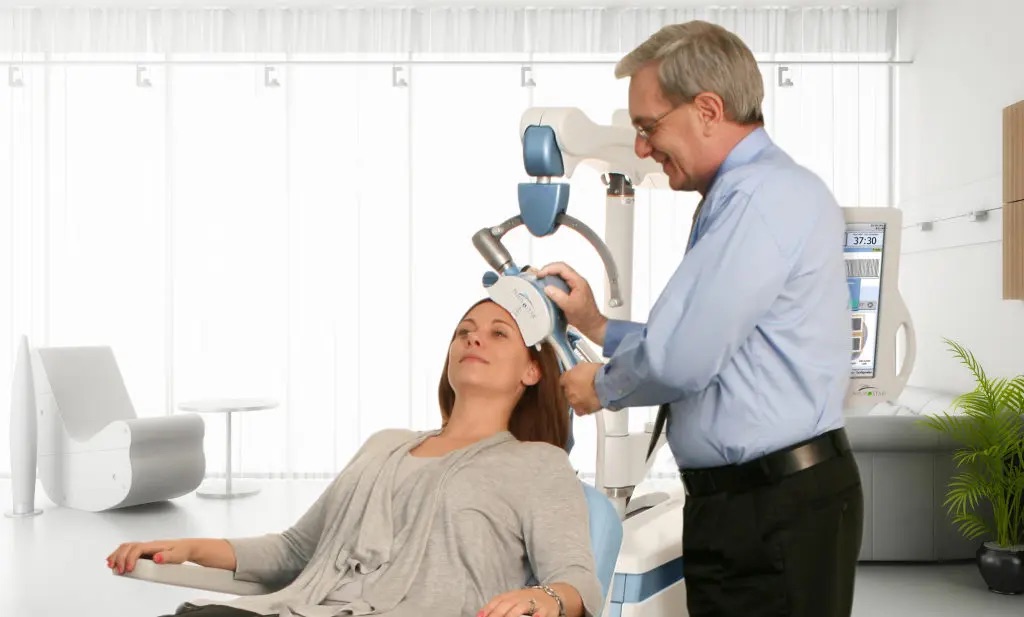
Transcranial magnetic stimulation (TMS) is a noninvasive procedure that uses magnetic fields to stimulate nerve cells in the brain to improve symptoms of depression. TMS is typically used when other depression treatments have not been effective. At NCBI, we are researching TMS as a treatment option for the following disorders;
Visual hemineglect from stroke or neurological disease
Parkinson’s disease
Neuropathic pain
Tinnitus